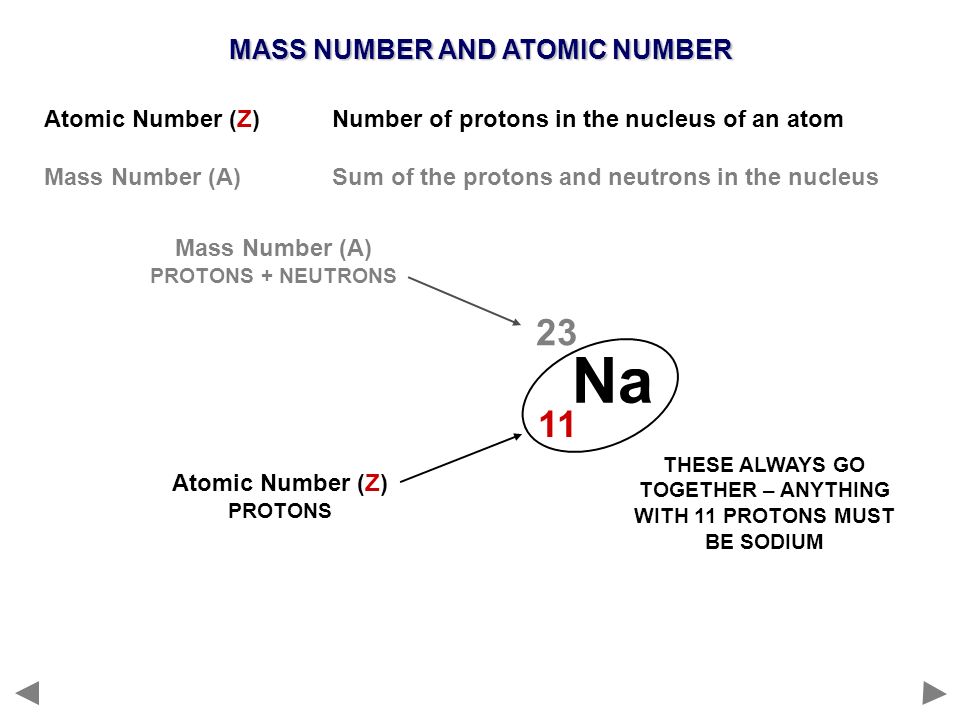
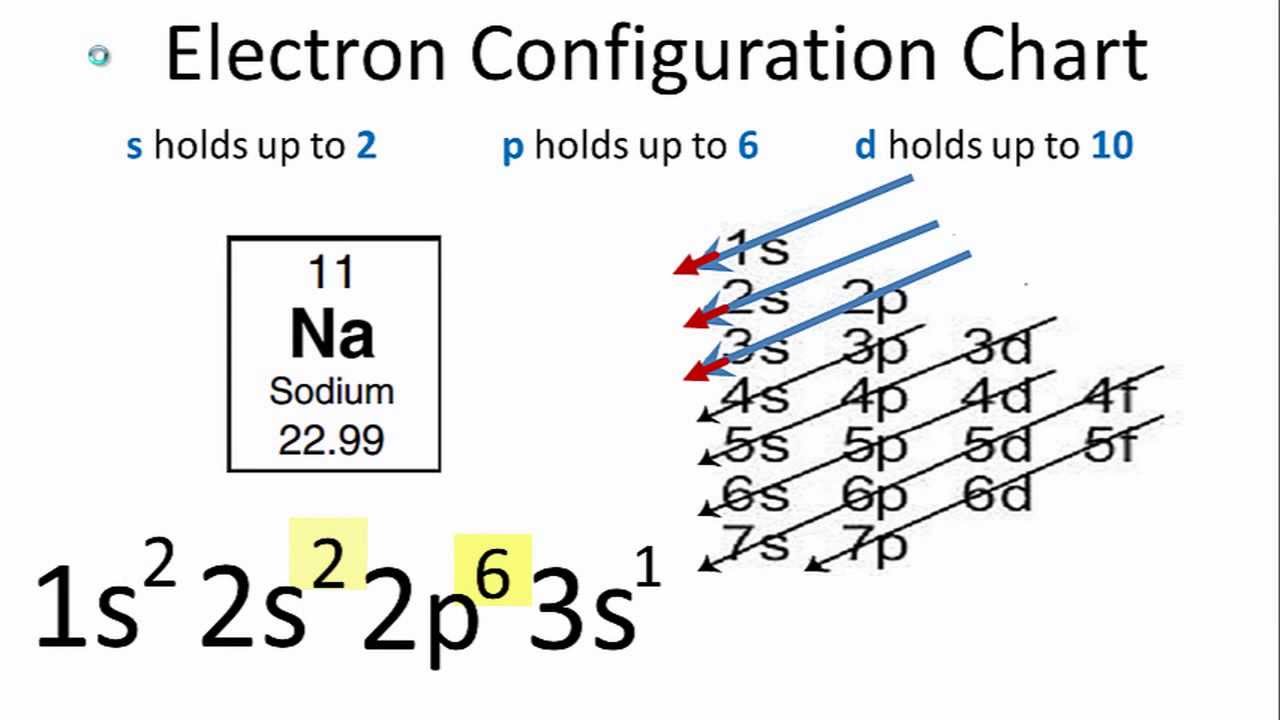
The first two fill the innermost energy level. A sodium atom contains 11 electrons orbiting a nucleus in 3 levels 2 in the lowest then 8 then 1 sodium has 11 electrons. When writing it the 38 goes directly underneath the 88.
The standard atomic notation for the most common form of strontium is 3888sr. Which is the same as 23 na because the 11 is redundant with the sodium symbol. We illustrate the notation for an isotope by showing the symbol for a sodium all sodium has z 11 nucleus with 12 neutrons.
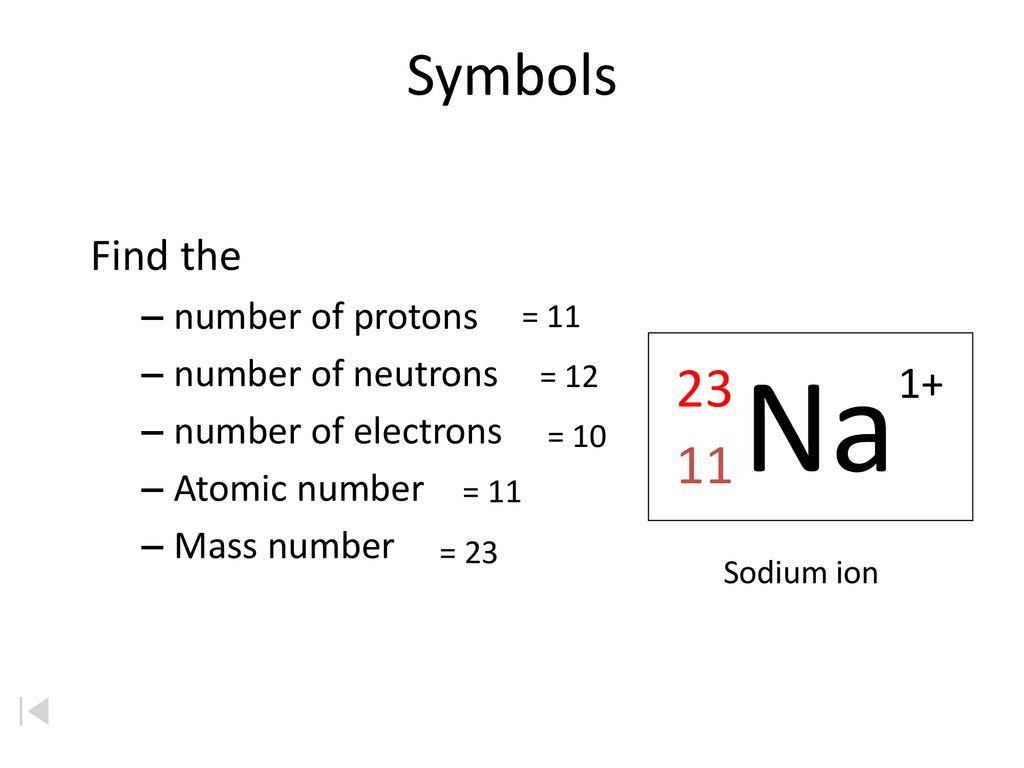
If two carbon nuclei have different number of neutrons one would say they are different isotopes of carbon. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotope s mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. And 24 na half life 15 hours.

It is considered a monoisotopic element and it has a standard atomic weight of 22 989 769 28 2 sodium has two radioactive cosmogenic isotopes 22 na half life 2 605 years. 23 na is the only stable and the only primordial isotope. There are 21 recognized isotopes of sodium 11 na ranging from 18 na to 39 na and two isomers 22m na and 24m na.

Which notation represents an atom of sodium with an atomic number of 11 and mass number of 24. Hope this helps 1. Na is the atomic notation of sodium.
Because of this reaction a sodium cooled reactor must have a second heat transfer loop so that radioactive sodium does not come in contact with the environment. Sodium 24 15 hour half life is limited in use by its short life and is produced by irradiation in a nuclear reactor. Other articles where sodium 24 is discussed.














